Subtopic 1: Purpose
The purpose of an asset is often a critical piece of information, although it may be obvious in many cases. For example it may have a purely promotional purpose (to sell a product to an appropriate audience), it may be educational, it may be for internal training, to increase awareness of a particular disease, to provide budgetary information for payers, or to provide support for patients.
The need to understand an asset’s purpose before approval is illustrated in the following examples and case studies.

Paracetanose

Consider the following (fictitious) statement which you can assume to be factually correct and capable of substantiation for the purposes of this exercise:
“Following the recent positive opinion by the CHMP, AstraZeneca expects Paracetanose™, an intranasal formulation of paracetamol for children aged 2-‐15 years, to be licensed and launched in Europe in 6 months time.”
Given the assumption that the statement is factual and it can be supported, it is still impossible to approve without knowing its purpose and how it is to be used. Here are some possibilities – click on those that you think it could be approvable for:
- Part of a presentation by an AZ medic during an advisory board Yes, this could be acceptable provided it was relevant in terms of the purpose of the advisory board and its attendees.
- Within a press release to the consumer media No, this would not be acceptable before the launch of the product – it may however be acceptable as part of a media launch campaign – this is covered in detail in the ‘working with the media’ module
- Within a press release to the financial media Yes, it could be considered acceptable to inform investors about positive opinions from regulatory authorities and launch plans – provided that the tone is not deemed promotional (see key topic 3 for more info).
- Within a value pack on Paracetanose for AZ companies Yes, it is acceptable to provide AZ companies with factual information on products prior to launch so that they can inform payers about cost effectiveness etc.
- Part of an internal briefing to the sales force who are expected to sell Paracetanose when it launches Yes, it is acceptable to tell company employees, including sales force about important company milestones. However it would be wise to include a statement for the sales force that they should not talk about Paracetanose with any customers prior to its launch.
- During a presentation given by an opinion leader at an AZ sponsored educational meeting about pain relief in children No, Educational meetings sponsored by AZ can potentially include factual information about its products if relevant to the meeting topic as a whole, but they must only cover licensed products and indications.
- Part of a disease awareness campaign about the burden of pain in children No, even licensed products cannot be specifically mentioned in disease awareness campaigns – see the module on ‘working with patients and the public’ for more detail.
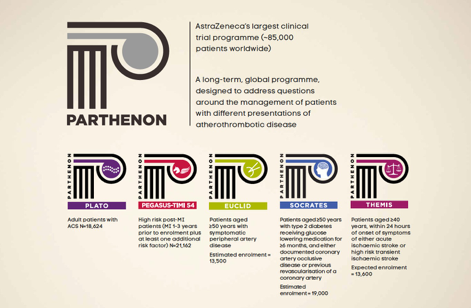
Parthenon programme
Consider the following panel about AstraZeneca’s clinical trial programme for ticagrelor. You can assume that all the information is factually correct.
For this exercise you also need to assume that the current licensed indication is as follows:
"Ticagrelor, co-‐administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with acute coronary syndromes (unstable angina, non ST elevation myocardial infarction [NSTEMI] or ST elevation myocardial infarction [STEMI]); including patients managed medically, and those who are managed with percutaneous coronary intervention (PCI) or coronary artery by-‐pass grafting (CABG)."
Assuming all obligatory information (such as job codes and dates etc) will be added as required, in which of the following ways would you approve it for use?
- On the clinical science area of an AZ stand at an international cardiology congress Yes, it is acceptable to share information about current clinical trial programmes at scientific congresses provided that it is not promotional (i.e does not make product claims and is separated from any promotional activities at the event)
- In a slide set for medical AZ staff to be used reactively in response to enquiries about Parthenon Yes, this would be acceptable when medical staff are responding to an unsolicited enquiry
- In a press backgrounder for medical journalists attending an international cardiology conference where AZ is exhibiting Yes, this is acceptable as this is medical journalists who are delegates at the congress, provided the information given is not promotional (see ‘working with the media’ module
- In a presentation for sales representatives to use to demonstrate AZ’s commitment to cardiology in general No, this is not acceptable because it contains information about unlicensed indications. If the information came from a sales representative it would be deemed as promotional and therefore ‘off licence’ promotion of ticagrelor.
- In a cardiology journal advertisement No, this would not be acceptable because journal advertisements are promotional and this contains information about unlicensed indications for ticagrelor.
- In an AZ sponsored symposium at a cardiology congress Yes, this could be acceptable at a genuine scientific congress provided that the symposium was part of the official congress programme (i.e. not a separate standalone event) and was not attended by sales representatives