Key Topic 7: Meetings and Hospitality
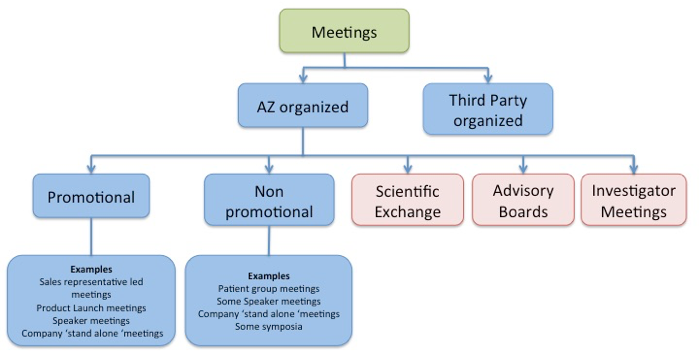
As you know there are many types of meetings that may require nominated signatory review and it is important that you establish which type you are dealing with e.g promotional or non promotional, scientific exchange etc.
This diagram may help you to slot a meeting into a particular category. You will notice that some types of meeting can fall into more than one category depending on their nature.
All meetings designed to update HCPs on areas of medicine, science or our products should have a clear educational content. It should enhance medical knowledge, enhance the proper use of medicines or enhance patient care.
Some of the key questions to ask yourself about meetings are:
- What is the type of meeting and purpose?
- Is there clear educational content where required?
- Are the hospitality arrangements acceptable?
- Is the impression of the meeting acceptable?
Other considerations:
- Is the documentation adequate?
- Are the costs acceptable?
- Are the content and arrangements appropriate for the target audience?
- Has the involvement of AstraZeneca been made clear?
Consider these examples:
Hospitality

The Medicines Australia Monitoring Committee referred hospitality provided by Takeda to healthcare professionals who attended the American Diabetes Association 74th Scientific Meeting for consideration by the Code Committee.
This was a 5 day event held in San Franciso in June 2014. The Monitoring Committee was concerned that the expenditure on alcoholic beverages at three dinners was high, out of proportion to the expenditure on food and was excessive.
Takeda had sponsored 5 delegates to attend the meeting. The company had provided international travel, accommodation, travel expenses and meals at a total cost of $53,723.
Provision of alcohol at the three meals was as follows (US dollars):
- Meal 1: wine $222 for 10 guests
- Meal 2: 4 bottles of wine at $320 for 12 guests (wine cost was 30% of total meal cost)
- Meal 3: spirits and wine costing $166 for 8 guests (>30% of total meal cost)
Do you think these costs are acceptable?
Ruling
The Code Committee noted that alcohol, especially wine, is considerably more expensive in restaurants in the United States than it is in Australia. A minority of members of the Code Committee, however, felt that regardless of higher costs at international restaurants, there had been less expensive alcoholic beverage options available at each of these restaurants, and there would have been more appropriately priced restaurant options available in San Francisco. However, a majority of the Committee members considered that the overall per head cost at each of the three dinners was acceptable and took into account the higher cost of alcoholic beverages, including wine, in US restaurants.
Hint and tip
AstraZeneca considers spirits and sparkling wines to be inappropriate hospitality
Venue
Would the following venues cause you some concern in terms of acceptability for a meeting organized or supported by AstraZeneca?
 The Plaza New York
The Plaza New York
Meetings facilities:
“The Plaza is proud to offer professional meeting facilities supported by a comprehensive array of professional and state-of-the-art services.”
Yes No
Answer
The hotel website also says: “The Plaza is the ultimate New York City luxury lifestyle destination - an iconic luxury hotel with a new and contemporary spirit.”
Others say: “Opened in 1907 and designated an official landmark in 1969, The Plaza is arguably the most famous hotel in New York.”
Although the hotel has good conference facilities, it is renowned as an ‘iconic hotel’ and would not be suitable on these grounds for an AstraZeneca organized or supported meeting.

Conference facilities at Birmingham City Football Club
Meetings facilities:
- Nine unique function rooms and ability to accommodate 350 delegates
- 17 executive private boxes
- City centre location
- Easy access from all major road routes
- 350 free car parking spaces
- Exceptional standards of amenities and service
- Award winning in-house catering
- Disabled access
- Complimentary Wi-fi
- Yes No
Answer
Acceptability would depend on a number of factors - for example it would be worth checking what ‘awards’ have been won for the catering etc. to decide whether it was ‘renowned’. However sports stadia often have large conference facilities and have been deemed acceptable in the past where it was shown that they were chosen on the basis of ease of location and delegate numbers. However for conference facilities associated with sports venues you should always check that there are no sporting events occurring at the same time as the meeting, as this could be viewed as the main attraction!
Note:
AstraZeneca does not allow meetings in restaurants in arenas or stadia, or museums.
Meeting purpose

In Australia the Medicines Australia Monitoring Committee review educational events conducted by pharmaceutical companies. As part of this review they asked for an event conducted by Amgen to be investigated.
Amgen had invited 14 health professionals who were delegates at the American Society of Nephrology (ASN) meeting in Atlanta to a tour of the company manufacturing facility in Puerto Rico.
Those invited were “opinion leaders in the nephrology community” who not only prescribed medicines for their patients but also were in influential positions as members of hospital or state formulary committees.
The event included 2 nights accommodation and 2 dinners. During the event presentations were given which provided clinical information about erythropoietin and the treatment of anaemia associated with chronic kidney disease and biosimilar medicines. Amgen marketed the product Aranesp. The cost of the meeting was just over $46,000.
Amgen stated that the meeting was organised because healthcare professionals needed to be assured through first-hand experience of a manufacturing facility that biologic medicines met appropriate quality standards.
Which of the following do you agree with?
- These presentations could have been given in Atlanta therefore the purpose of the meeting in Puerto Rico is not acceptable
- The hospitality is out of proportion to the educational content
- $46,000 for 14 health professionals is excessive
- This meeting would not stand up to public scrutiny
- The reason for this meeting is unconvincing
Ruling
The Committee found this event to be in breach of the Australian Code. They were ‘very concerned that the primary purpose of the meeting was to influence individual healthcare professionals to prescribe Aranesp and more broadly to influence key decision makers who are members of hospital formulary committees. They considered that the primary purpose of the presentations and the plant tour were to persuade the attending healthcare professionals to continue to prescribe and recommend Aranesp rather than any biosimilar product. In conclusion the educational content would not stand up to public scrutiny. However the travel and accommodation provided was appropriate.
Amgen were fined $200,000.
Interestingly this ruling was overturned on Appeal. In terms of educational content the majority of the Appeals Committee accepted that it was beneficial for specialist physicians to be educated about these complex molecules and the differences between biologics and small molecule medicines.
In addition the majority of the Committee accepted that it was reasonable to hold the educational meeting at the Puerto Rico plant following the ASN meeting in Atlanta, as the delegates were in relatively close proximity.
This case demonstrates that these matters are often a matter of judgement.
Educational content
In the UK an anonymous complainant provided some photographs which were said to show a Merck Sharp & Dohme sales representative entertaining a group of doctors and their wives at a Chinese restaurant. The complainant alleged that the meeting had no educational content and was held in the public domain.
The Panel examined the restaurant receipts and found the costs to be acceptable. However there was no written invitation, no agenda and little other information. The meeting did not have a sufficiently clear educational content to justify the provision of hospitality. The meeting had been held on a Friday night in a part of a restaurant where the public were also present. The venue was therefore deemed unsuitable. The representative had not maintained a high standard of ethical conduct. The Panel considered that the arrangements for the meeting were totally unacceptable. MSD was found to have brought discredit upon the industry.

This example illustrates the importance of documentation, the need for clear educational content, acceptability of venue and the general ‘impression’ created which the Panel said was “a mainly social event on a Friday night paid for by the pharmaceutical industry.”
Suitability for sponsorship

In the UK a group of health professionals complained about sponsorship of a meeting of the Irish Society of Urology by several pharmaceutical companies. The meeting was held in the UK.
The first page of the scientific programme featured photographs of the very luxurious, 5 star venue and nearby attractions. The welcome message on the first page of programme read:
‘The social aspect of this meeting is extremely important and the two evening events promise great enjoyment. The unique opportunity to have our gala dinner in Stormont was one that we couldn’t pass over!’
Most of the second day of the meeting was dedicated to playing golf and leisure activities as clearly marked in the programme. However the delegates themselves paid for the golf.
On the back of the programme one of the companies had made this declaration:
An educational grant was provided by Allergan Ltd to the Irish Society of Urology to support this independent course. Allergan has had no involvement in the logistics, design or content of the course.
The other sponsoring companies were just listed.
Which of the following do you agree with?
- This venue cannot be used for this meeting as it is lavish and 5-star the Irish Society of Urology can hold its meetings wherever it wishes. However the question is whether that then makes it unsuitable for sponsorship by a pharmaceutical company.
- The declaration by Allergan makes their lack of involvement clear and so their sponsorship of this meeting is acceptable whilst it is true that company involvement should be made clear, this statement could not absolve Allergan of responsibility.
- Pharmaceutical companies should avoid sponsoring meetings such as this the venue alone would make this unacceptable, but there is almost a whole day of golfing activity which brings into question the balance of educational content etc.
- It is unwise to put images and descriptions of venues on meeting invitations since it is the educational content of a meeting that should be the attraction, there should not be need to describe the venue and its facilities in detail
Ruling
Taking all the circumstances into account it appeared to the Panel that the pharmaceutical companies listed on the back page of the programme had supported all the arrangements for the two-day meeting held at a luxurious venue with golf and a gala dinner. The Panel considered that the arrangements for the meeting as described in the programme and the impression given were unacceptable. The companies were ruled in breach of the UK Code.