Key Topic 2: Promotional Claims
We will now turn our attention to promotional claims.
As you know there are some fundamental requirements that apply to all promotional claims, and good judgment is needed to ensure that all requirements are met all of the time.
Always keep in mind that imagery and artwork can constitute a claim as well as text.
For this reason its worth taking a ‘helicopter view’ of an asset in terms of layout, imagery, overall impression etc before getting into the detail of the claims themselves.
In addition high standards are expected in promotion at all times – so consider whether any content or format could cause offence.
This pie chart from 2013 shows the top reasons for FDA warning letters about pharmaceutical advertisements, so keep these in mind as you go through the examples in this course.
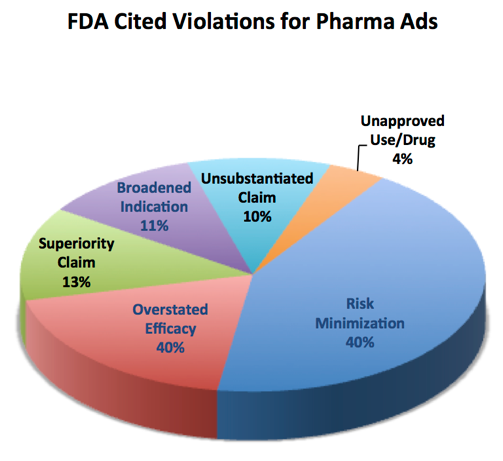
The requirements for promotional claims that we will now consider are:
Claims must be accurate and fair
A leave-piece produced by GSK in the UK for Seretide (salmeterol/fluticasone) was headed

“Cost comparison for combination therapies in asthma at beclometasone equivalent daily doses”.
Under this heading was a chart comparing various combinations and doses. The comparisons were grouped according to low, medium and high dose beclometasone. The cost per 30 days’ treatment at sustained dosing was given and the final column of the chart was headed ‘Cost difference with Seretide per 30 day treatment’.
There was an associated claim that stated: Seretide can be up to £35.08 cheaper for 30 days treatment at a stable dose than Symbicort (budesonide/formoterol). In the chart the difference of £35.08 compared 30 days of Symbicort 400/12, two puffs bd vs 30 days of Seretide 500 Accuhaler, one puff bd.
Do you think this comparison is accurate, fair and balanced?
Hint – you may need to refer to the SmPCs for both products.
YES
AstraZeneca complained that this was an unfair comparison because the Symbicort 400/12 summary of product characteristics stated that the recommended dose was one puff bd. Although some adults might require up to two puffs bd, very few prescriptions were for Symbicort 400/12, two puffs bd.
On this basis the comparison was deemed unfair by the UK Authority as it exaggerated the cost difference between the two products and was not a fair reflection of all of the data.
Claims must be balanced
Consider the following tweet (written by a celebrity) that was posted on Twitter by American company Duchesnay Inc.
They market the product DICLEGIS (doxylamine succinate and pyridoxine hydrochloride):
OMG. Have you heard about this? As you guys know my #morningsickness has been pretty bad. I tried changing things about my lifestyle, like my diet, but nothing helped, so I talked to my doctor. He prescribed me #Diclegis, and I felt a lot better and most importantly, it’s been studied and there was no increased risk to the baby. I’m so excited and happy with my results that I’m partnering with Duchesnay USA to raise awareness about treating morning sickness. If you have morning sickness, be safe and sure to ask your doctor about the pill with the pregnant woman on it and find out more www.diclegis.com; www.DiclegisImportantSafetyInfo.com.

The US PI for DICLEGIS contains the following important information:
DICLEGIS is indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management. Limitations of Use DICLEGIS has not been studied in women with hyperemesis gravidarum.
It also includes Warnings and Precautions regarding activities requiring mental alertness and concomitant medical conditions. In addition, the most common adverse reaction reported with DICLEGIS was somnolence.
Which of the following do you agree with?
- This is approvable as they have included a link to the important safety information
- This tweet is not balanced as it omits important safety information
- Omitting safety information in a tweet is approvable due to the word limit on tweets
- This tweet misleads the reader into thinking that DICLEGIS is safer than has been demonstrated
- This tweet is approvable as promotion to the public is allowed in the US
Ruling
This tweet was the subject of an FDA warning letter because of the lack of balanced information that made it misleading. FDA said it was misleading as it left out all safety information about the product as well as the material fact that the product had not been studied in women with hyperemesis gravidarum. The link to the safety information did not mitigate the misleading omission of risk information. A number of sanctions were imposed including issuing corrective tweets.
Claims must not mislead
MSD were found in breach of the Australian Code of Conduct for a misleading representation of data for their product Sevikar (olmesartan and amlodipine). The claim at issue was:
“Power to reach BP target in 7 out of 10 patients”
The data to support the claim were from the AZTEC study of Punzi et al 2010. A graph showed only those patients in the study that were up-titrated to the combination therapy (Sevikar) and omitted a histogram representing those patients who had achieved the blood pressure (BP) target on monotherapy (amlodipine) alone. There were 23.8 percent of patients who achieved the target BP on amlodipine alone and these were a subset of the total of 76.8 percent of patients who reached the BP target.
The Committee determined that the claim was misleading because it gave the impression that the outcome of 7 out of 10 patients achieving BP target was achieved only in patients using Sevikar combination therapy whereas this result was for patients on amlodipine monotherapy combined with those on the combination therapy.
Claims must be up to date
In the UK Wyeth complained about claims that appeared in a Roche media statement because they were incorrect and did not reflect an up to date view of all the evidence.
The claims were:
‘New Data Reveals Tocilizumab Is The First And Only Biologic Drug To Show Superiority Over Current Standard Of Care In Rheumatoid Arthritis’
and
‘No previous biologic therapy has demonstrated superiority compared to [methotrexate] MTX’
The statements implied that no other biologic agents had been shown to be superior to methotrexate. However Wyeth showed there was a wealth of evidence supporting the superiority over MTX of other biologic agents with existing marketing authorizations and so the claims were ruled in breach.
Claims must not be inconsistent with the details of the marketing authorization
Below are some claims in a sales aid for Tindamax in the US.
This agent is licensed in the US as follows:
Tindamax is also indicated for the treatment of bacterial vaginosis (BV) in non-pregnant women; pathogens such as Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, Candida albicans, and Herpes simplex virus should be ruled out.
There is also a boxed warning for the product for the potential risk for carcinogenicity, as well as contraindications for use in patients with previous hypersensitivity to tinidazole or other nitroimidazole derivatives, during the first trimester of pregnancy, and in nursing mothers.
Claims:
“TINDAMAX (tinidazole tablets) is the one and only treatment for BV that gives you patients...”
“For short, affordable treatment of bacterial vaginosis (BV)..”
Which of the following do you agree with?
- The claims are not inconsistent with the licensed indication
- The claims are inconsistent with the licensed indication
- The claims are misleading
- The claims are not approvable as they could compromise patient safety
Ruling
These claims miss out an important part of the licensed indication – it is licensed in non-pregnant women. Moreover there is a boxed warning about use in early pregnancy. They misleadingly imply that TINDAMAX can be used in any patients with BV and therefore the claims are inconsistent with the marketing authorization. The claims were the subject of an FDA warning letter because of this, and the company was told to cease use of the claims.
Claims must support rational prescribing and must be substantiated
Take a look at the following promotional leavepiece for a topical oestradiol gel (EstroGel promo material) and the boxed warning in the prescribing information for this product.
Which of the following do you agree with?
- There is appropriate balance of safety and efficacy information in this piece
- The important risks of cancer are mentioned in the piece so this is approvable
- The piece refers the reader to the boxed warning and PI so this is approvable
- The omission of risk information on this piece makes it potentially misleading
Ruling
This was the subject of an FDA warning letter due to the omission of important risk information. Although the piece contained the statement ‘estrogen therapies increase the risk of certain cancers, cardiovascular disorders and probable dementia’ the FDA said that the piece failed to disclose material information about specific cancer and cardiovascular risks in the boxed warning. The fact that the reader was referred to the PI and boxed warning did not mitigate the omission of these important risks.
As you will remember health professionals should not have to ‘dig out’ the important information – it should be made easy for them to take the balanced view from materials without a lot of extra work on their part.

