Substantiation
All statements that you make about medicines or therapy areas must be supportable by some kind of evidence. You and your signatory will need to consider the quality of evidence that is supporting a statement.
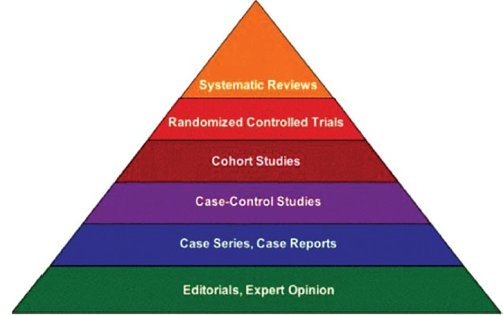
You may be aware of the ‘hierarchy of evidence’ shown here. Generally data from systematic reviews and controlled clinical trials are considered to provide stronger evidence than case studies or anecdotal evidence. ‘Real World’ studies are somewhere in between.
The standard of evidence required to support a particular statement will depend on the nature of the statement and the therapy area. In an area that has been well studied, systematic reviews may be easy to find. However in some therapy areas such as very rare conditions the totality of evidence may be from layers lower down in the evidence hierarchy.
Materials based upon ‘real world’ evidence should include a statement making it clear that the information is based on real world evidence and is therefore subject to potential bias. For real world studies the term ‘associated with’ should be used to describe findings as opposed to saying a study ‘showed’ a particular effect e.g. In a real world study, using observational data obtained from patients electronic health records, Product X was associated with a reduced annual rate of exacerbations of COPD compared to Product Y.
When statements refer to published studies clear references should be given in the material. If using unpublished data to support a claim you will need to construct a ‘data on file’ document so that your signatory can verify the substantiation of the claim. Refer to the ‘Guidance for Creation of Global Data on File Packets (DoFP))’ for details of how data on file packets are produced and approved, as well as the acceptable format.
Consider these examples
Superiority statements
In the US Cipher Pharmaceuticals sent a promotional email about their product LIPOFEN (fenofibrate). The contents of the email were as follows:

Title: e-pharm alert: All fenofibrates are not created equal
When a patient is prescribed LIPOFEN (fenofibrate capsules USP) a generic fibrate may not be the best option. Only LIPOFEN offers Lidose technology which:
Uses a unique lipid melt matrix system not available with any other generic or brand name fibrate;
Delivers reliable, consistent and uniform delivery;
Avoids dependence on particle formulation;
May improve the safety and efficacy of the active ingredient in LIPOFEN by offering more consistent plasma profiles
LIPOFEN Takes Particle Size Out of the Equation;
Other fenofibrates are formulated with small particles, which may affect absorption;
With LIPOFEN, particle size is not an issue;
Fenofibrate is in an already-dissolved state, making it readily available for absorption;
LIPOFEN with Lidose technology offers a very homogeneous distribution of fenofibrate in the mass of excipients;
No crystals of fenofibrate are observed.
Assuming that the facts about the formulation of LIPOFEN are true, and that appropriate obligatory information was included, which of the following do you agree with?
- If factually accurate this email is approvable
- This email makes superiority statements versus all other fibrates
- To substantiate these statements I would need to provide results of head to head studies against other fibrates
- If there were no head to head data against other fibrates this email would be misleading
Ruling
This email was the subject of an FDA warning letter. The FDA stated:
‘In general, claims of superiority must be supported by adequate and well-controlled head-to-head clinical trials comparing appropriate doses and dose regimens of your drug and the comparator drugs. Although we acknowledge that some of the above claims may be true; the totality of the claims and presentations misleadingly implies that Lipofen offers a clinical advantage over other fenofibrate products.’
(There were no head to head comparative data to support the implication of superiority)
Product adherence statements
DaraBiosciences Inc made the following statements with respect to their oral formulation of tamoxifen (Soltomox) in the US:
CHALLENGE:
Compliance and adherence to prescribed tamoxifen therapy is a critical issue in patient care and may affect treatment outcomes. Difficulty swallowing pills may affect both compliance and adherence to prescribed courses of oral solid medications.
SOLUTION:
Liquid medication may be preferred vs. pills by many patients
- Many adults in the USA were reported to have experienced difficulties with swallowing tablets or capsules at some point, even though most had no problems swallowing food or liquid
- Research shows that women experience more discomfort with pill swallowing compared to men
- Many adults who have trouble ingesting their medication have not discussed the situation with a health professional
Soltomox, the only tamoxifen citrate oral solution that may support long-term adherence.

Assuming the bullet points are true and can be substantiated, which of the following statements do you agree with?
- The final statement is supportable because they say it ‘may’ support long term compliance
- The final statement needs to be supported by some clinical data
- Without supporting clinical evidence for the final statement it cannot be approved
- The evidence on compliance issues with tamoxifen tablets would be suitable to support the final statement
Ruling
These statements were the subject of an FDA warning letter. The FDA stated “materials are false or misleading if they represent or suggest that a drug is safer or more effective than another drug, when such has not been demonstrated by substantial evidence or substantial clinical experience.
It was considered that the statements suggested that, as a result of its liquid dosage form, Soltamox offers a therapeutic advantage over other available formulations of tamoxifen. There were no studies that specifically evaluated the liquid formulation of tamoxifen with respect to compliance. Therefore the statements could not be substantiated.

