Historical Perspective
The modern pharmaceutical industry is highly regulated but the various laws and codes that are in place today, have evolved fairly recently (since the early 1900s), and in some cases they were introduced in response to some tragic events worldwide.
The regulations governing promotion of medicines are even more recent, and they continue to evolve. Keeping up to date with these developments is one of the key requirements for medical personnel.
Click on the links below for some examples of historical events that have influenced current pharmaceutical regulations and examples of promotion of medicines that would definitely not be allowed today!
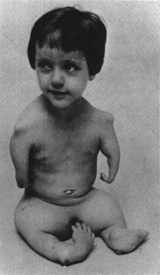
The Thalidomide Disaster
The first paper describing the pharmacological effects of thalidomide was published in 1936. It was the only non-‐barbiturate sedative available at that time. It entered the German market in 1957 as an over-‐the-‐counter sedative that was ‘particularly suitable for infants and the elderly’. It was advertised as ‘safe’ and ‘free from untoward side effects’.
It was soon discovered that thalidomide could also relieve morning sickness in pregnant women although this was an unlicensed indication. By 1960, thalidomide was marketed in 46 countries, with sales nearly matching those of aspirin. Notably it was refused a licence in the US due to lack of clinical evidence about its side effects. By 1960 doctors had expressed concerns over its teratogenic effects – in particular severe limb deformities in babies born to mothers who had taken it during pregnancy. It was withdrawn in 1961 after which the regulations were changed to ensure that any new drug was screened for the potential to cause harm to the unborn baby.
Advertising of heroin (diamorphine)
Diacetylmorphine (diamorphine) was first synthesized in 1874 by Charles Romley Alder Wright, an English chemist. He had been experimenting with combining morphine with various acids and produced a more potent, acetylated form of morphine.
From 1898 through to 1910, diacetylmorphine was marketed by Bayer under the trade-‐name ‘Heroin’ as a non-‐addictive alternative to morphine and cough suppressant. By 1899, Bayer was producing about a ton of heroin a year, and exporting the drug to 23 countries. There were heroin pastilles, heroin cough lozenges, heroin tablets, water-‐soluble heroin salts and a heroin elixir in a glycerine solution.
"It possesses many advantages over morphine," wrote the Boston Medical and Surgical Journal in 1900. "It's not hypnotic, and there's no danger of acquiring a habit.”
In 1913, Bayer decided to stop making heroin when its addictive properties were recognized. There had been an explosion of heroin related hospital admissions in the US and a large population of recreational users was reported. The next year the use of heroin without prescription was outlawed in the US. (A court ruling in 1919 made it illegal for doctors to prescribe it to addicts.)
Heroin was first restricted in the UK in 1868 due to 'unprofitable diversion of workers' and an international ban followed after the first World War.
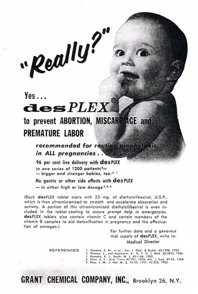
The diethylstilbestrol (DES) story
In the 1940s, the synthetic oestrogen, diethylstilbestrol (DES), was advertised around the world to “prevent miscarriages” and in healthy pregnancies “to make babies stronger”.
The advert shown here states that it was recommended for routine prophylaxis in ALL pregnancies, and that it had ‘no gastric or other side effects’.
Unfortunately women who took it ended up with a higher risk of breast cancer and their daughters, exposed in utero, developed reproductive tract abnormalities and, in some cases, a rare form of vaginal cancer.