Key Topic 1: Encourage rational prescribing
The historical examples in the ‘principles of review’ module serve as a reminder that patient safety should underpin every communication from pharmaceutical companies to health professionals.
Health professionals have a duty to stay up to date with developments in their field, and often developments will include new medicines becoming available or new uses for existing medicines. Whilst information about medicines in terms of effectiveness and safety can come from published clinical trial data, health professionals often need information that isn’t necessarily published to assist them in their decision making.
It is important that health professionals can trust that the information they receive from pharmaceutical companies is accurate, up to date and not misleading in any way.
In addition, the regulatory authorities tend to take the view that a health professional should not have to ‘dig’ for information within pharmaceutical materials in order to get a balanced message. Therefore beware of small footnotes and remember that a link to prescribing information does not always suffice in terms of providing the necessary information.
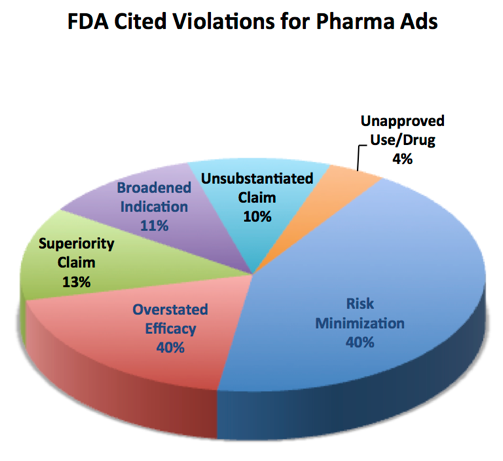
The pie chart from 2013 (on the right) shows the top reasons for FDA warning letters about pharmaceutical advertisements.
We will look at some examples of these sorts of ‘violations’ both in and outside of the US and how they may undermine ‘rational use’ of a medicine.

